Describe the Orbitals Used by the Carbon Atom in Bonding
A CH 3-CH 3 b CH 3-CH CH 2 c CH 3-CH 2-OH d CH 3-CHO e CH 3 COOH. 15 Describing Chemical Bonds.

2 2 Hybrid Orbitals Organic Chemistry 1 An Open Textbook
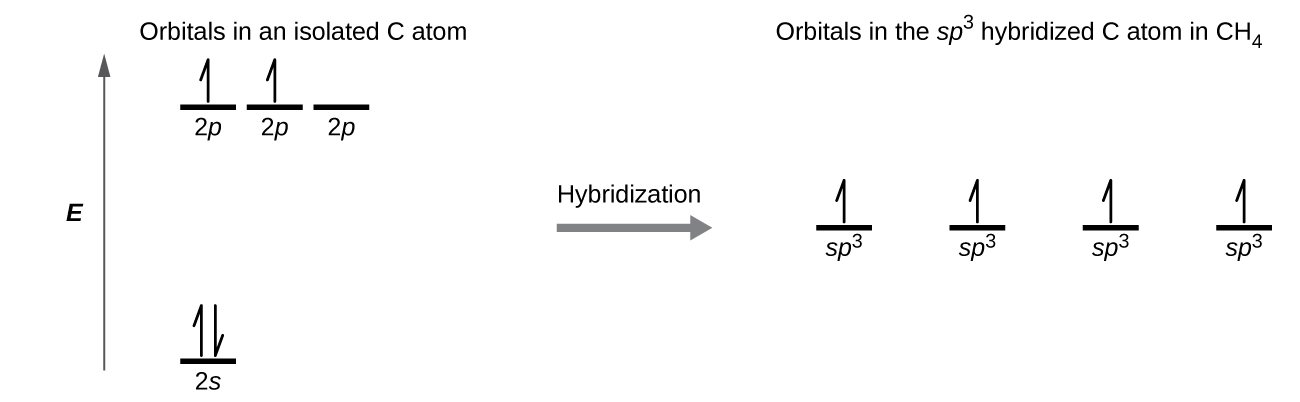
This creates four equivalent sp 3 hybridized orbitals.

. The four valence atomic orbitals from an isolated carbon atom all hybridize when the carbon bonds in a molecule like CH 4 with four regions of electron density. Electrons are paired in the overlapping orbitals and are attracted to nuclei of both atoms HH. Illustrate by giving one example of each type.
Singly occupied orbital on the other atom Two models to describe covalent bonding. The carbon atom can use its two singly occupied p-type orbitals to form two covalent bonds with two hydrogen atoms yielding the singlet methylene CH 2 the simplest carbeneThe carbon atom can also bond to four hydrogen atoms by an excitation or promotion of an electron from the doubly occupied 2s orbital to the empty 2p orbital producing four singly occupied orbitals. Explore molecule shapes by building molecules in 3D.
What do you understand by bond pairs and lone pairs of electrons. Valence electrons not used in bonding are called nonbonding electrons. Valence bond theory Molecular orbital theory.
Overlap of each of the hybrid orbitals with a hydrogen orbital creates a CH σ bond. Valence Bond Theory. Which hybrid orbitals are used by carbon atoms in the following molecules.
How does molecule shape change with different numbers of bonds and electron pairs. Then compare the model to real molecules. The electron pair involved in sharing between two atoms during covalent.
In a methane molecule the 1s orbital of each of the four hydrogen atoms. Find out by adding single double or triple bonds and lone pairs to the central atom.

2 2 Hybrid Orbitals Organic Chemistry 1 An Open Textbook
8 2 Hybrid Atomic Orbitals Chemistry

Which Hybrid Orbitals Are Used By Carbon Atoms In The Following Molecules A Ch 3 Ch 3 B Youtube
No comments for "Describe the Orbitals Used by the Carbon Atom in Bonding"
Post a Comment